
If you are a Teacher of Chemistry and would like to make a variation of any of the games presented on this website, please send an email to lbhsic2020@gmail.com to initiate a collaboration with lead programmer Steve Sogo. Steve will offer a workshop on using Unity to animate organic reaction mechanisms at BCCE 2026!
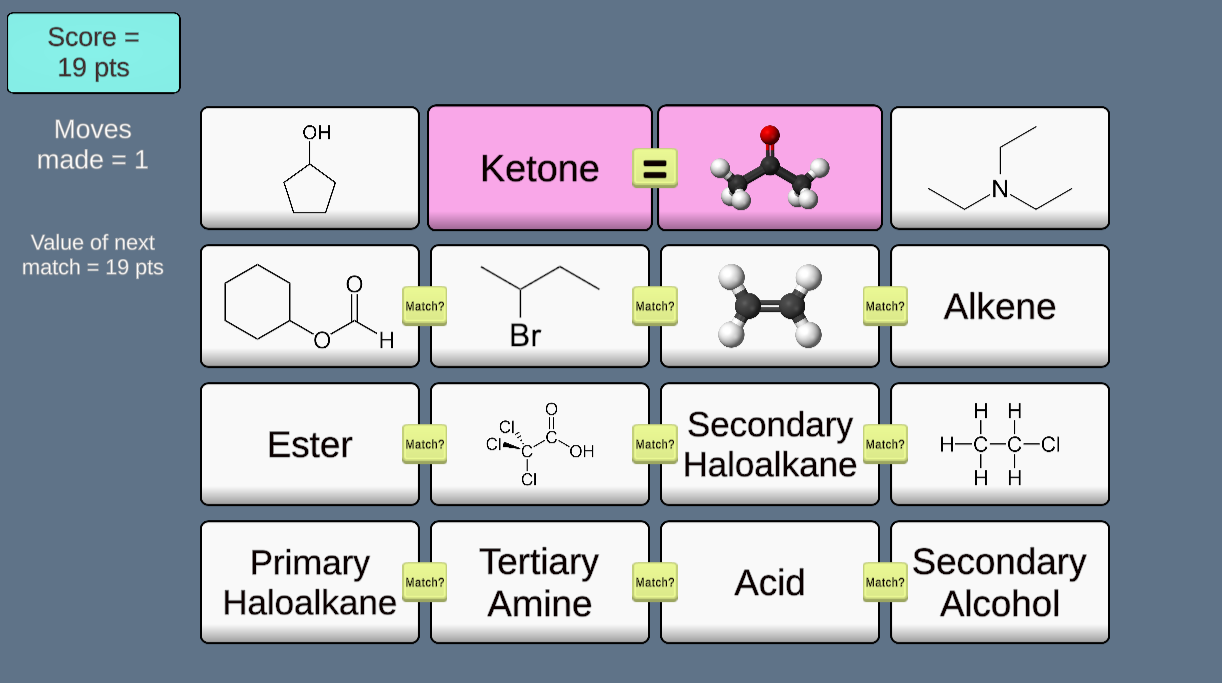
This game (inspired by teacher Matthew Smith of London, England) provides a playful way to learn (or review) families of organic compounds by matching molecular structure tiles to corresponding categories. Every run provides a randomized setup, so the game never plays the same twice!
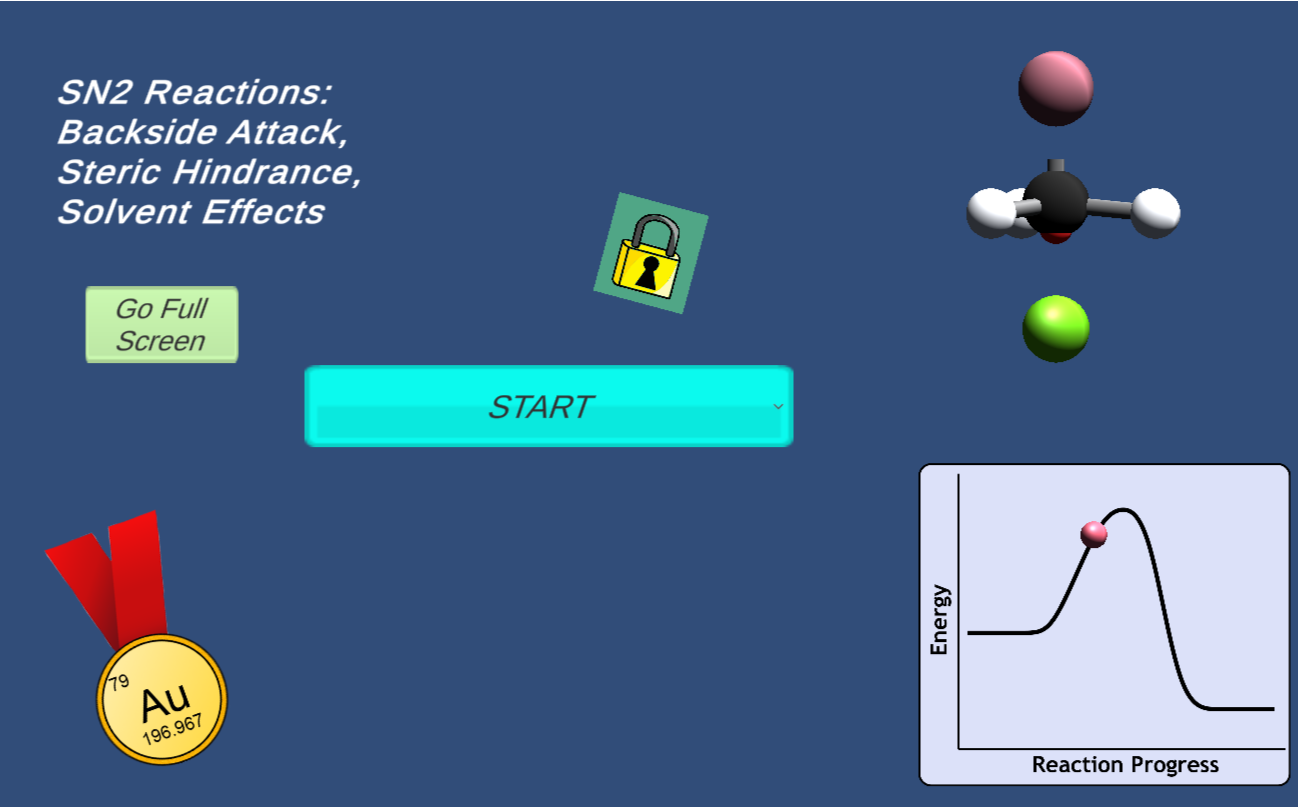
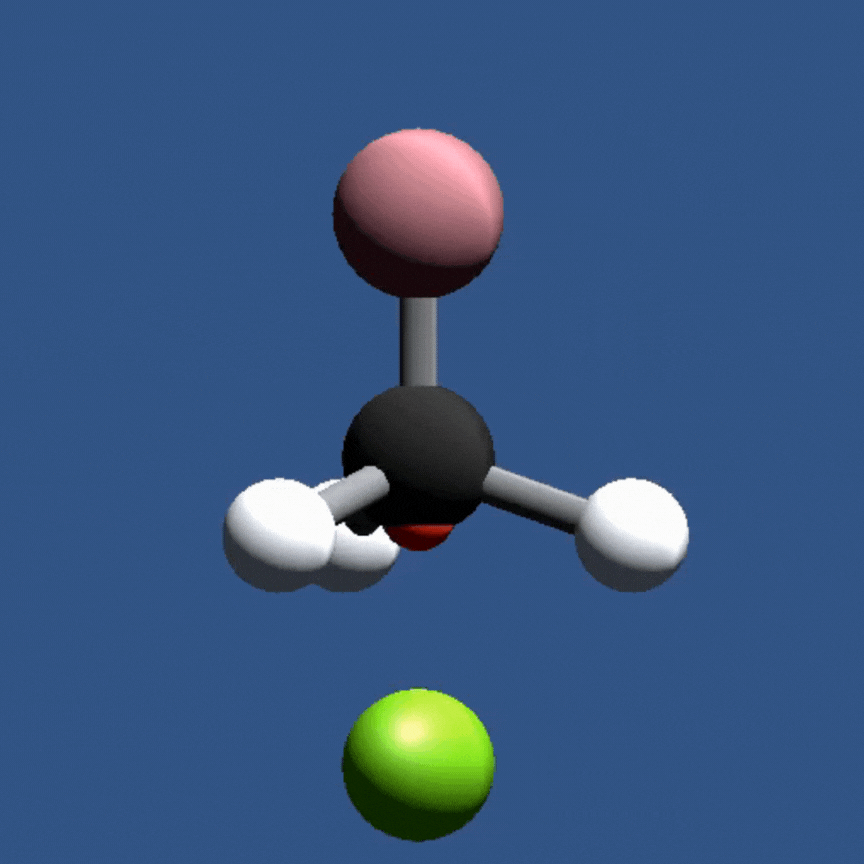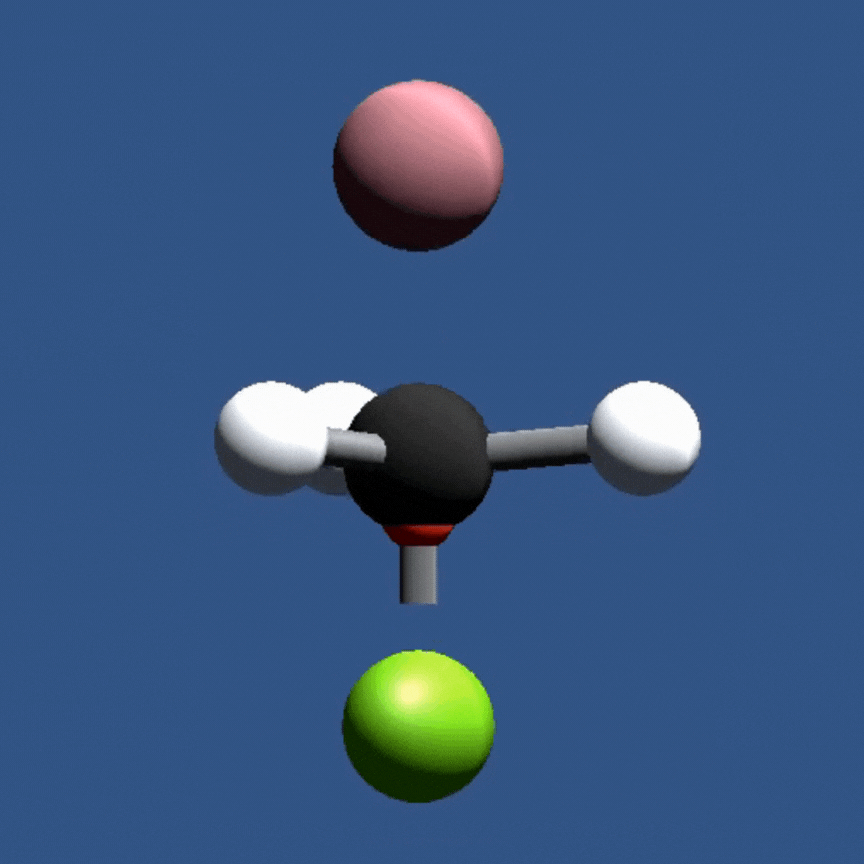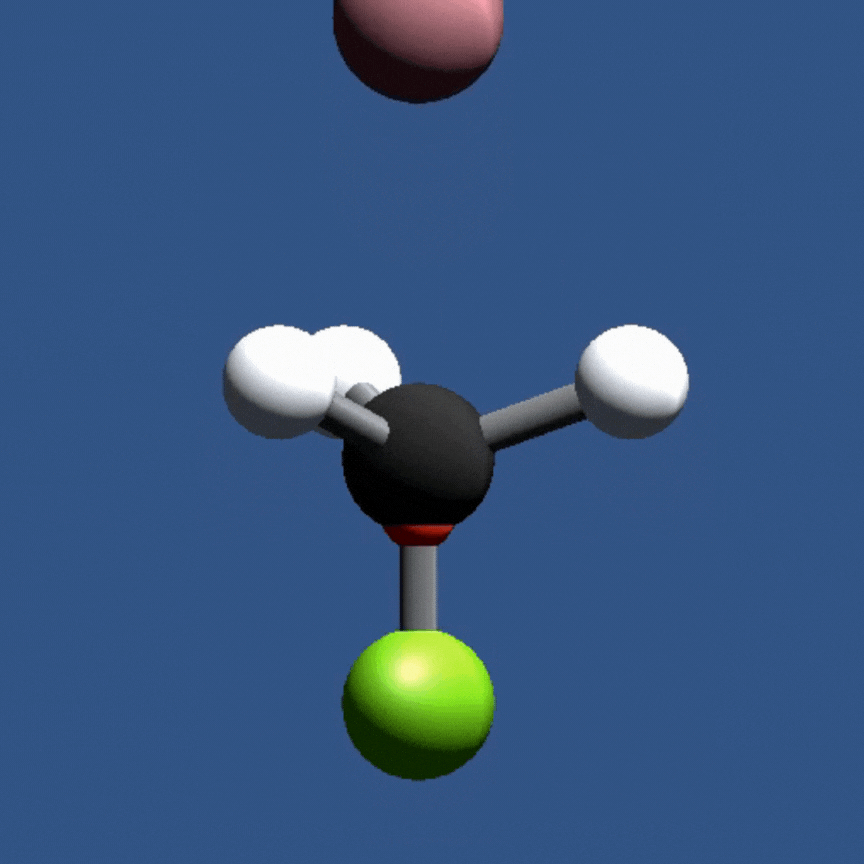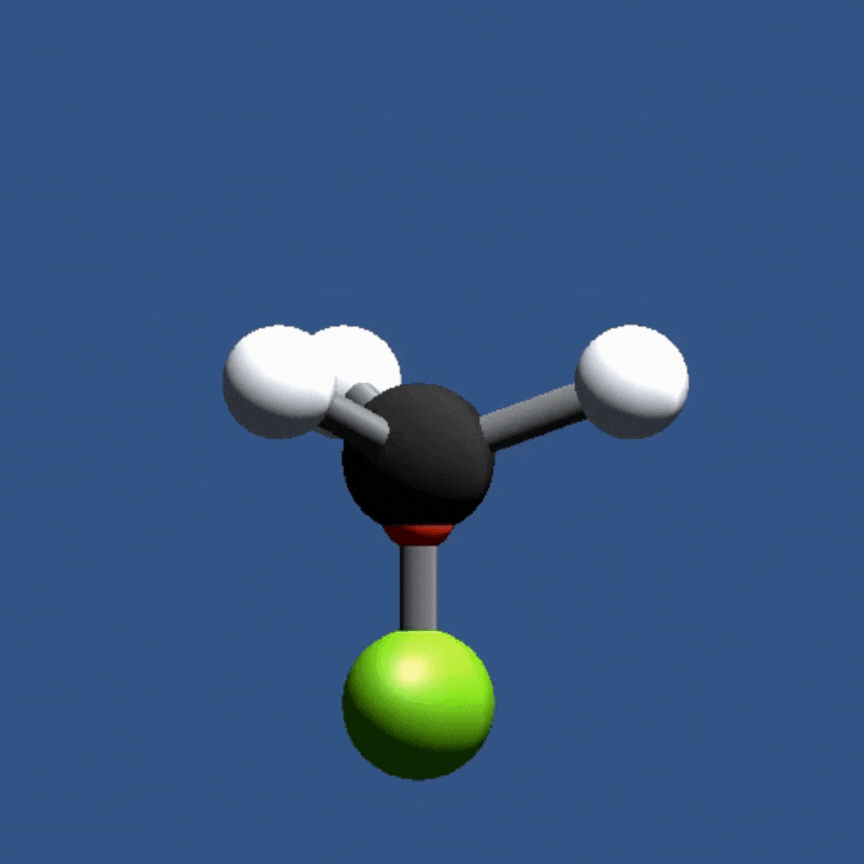
In this game, students will fire a nucleophile at a rotating electrophilic target.
Precise alignment of the nucleophile is required for a successful reaction. The concept of
activation energy and solvent effects are also introduced in a fun and playful manner.
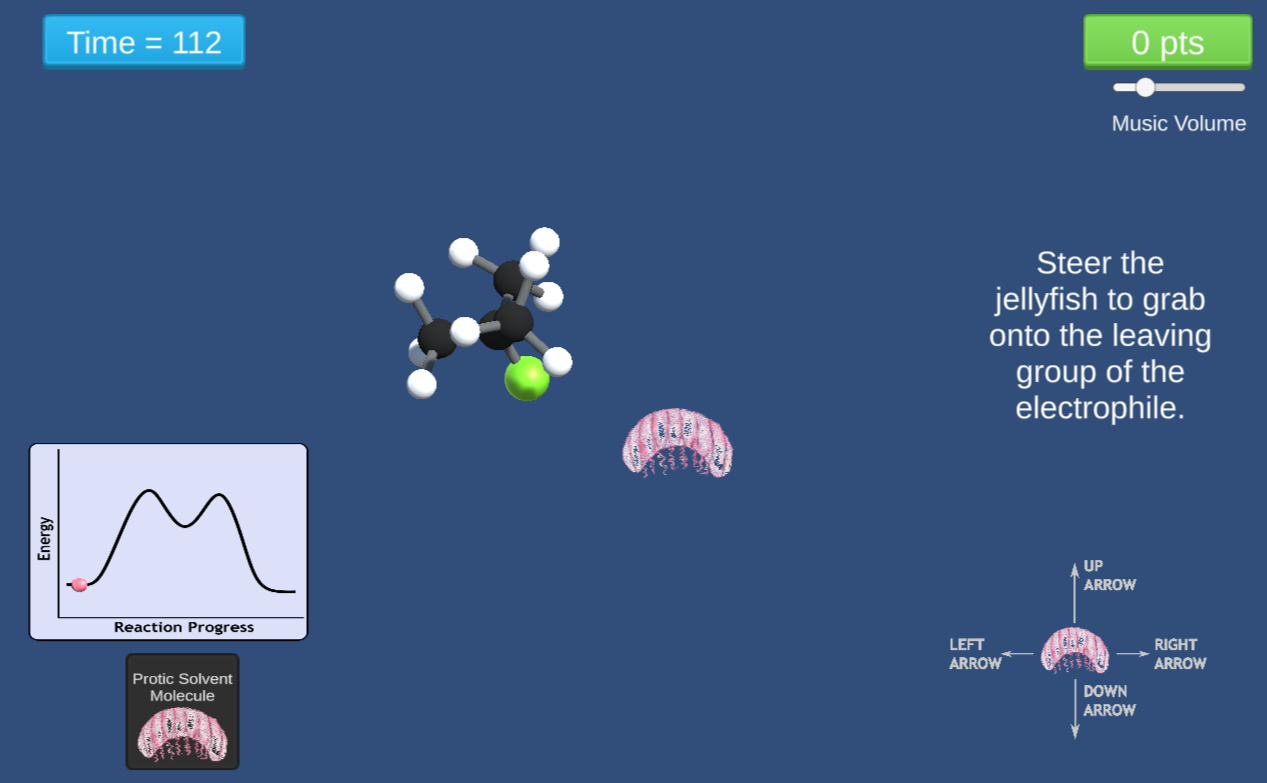
In this game, students steer molecules using a PacMan like interface to investigate multi-step substitution reactions. The games feature solvent-induced removal of a leaving group, formation of an unstable carbonium ion, and deprotonation steps.

This row highlights newly developed games featured at the ChemEd2025 conference held at Colorado School of Mines July 20-24. By playing these games, students will have fun learning the method of Dimensional Analysis and investigating interactions of atoms, ions, and molecules governed by the laws of physics.
 `
`
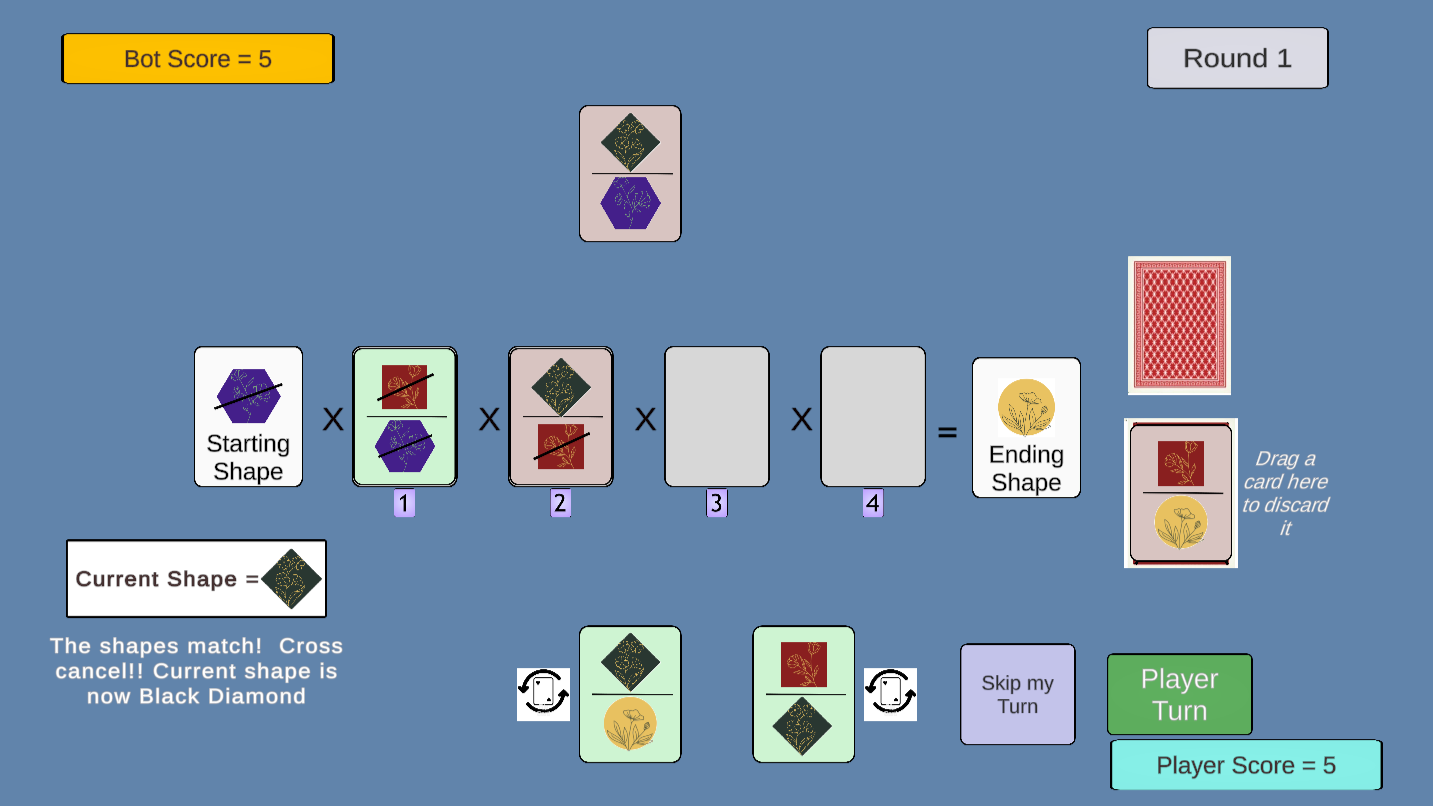
This game (inspired by teacher Ricky Tam of Calgary, Canada) provides a playful introduction to the method of dimensional analysis (unit conversion) used in chemistry classrooms. The introductory game uses a deck of cards with colorful shapes and provides a bot opponent. Later rounds introduce units such as grams, moles, Liters, etc.
.png)
In this game, students will solve puzzles with charged particles, gaining a feel for how Coulomb's Law (attraction and repulsion of charged particles)
can move ions in desired directions.
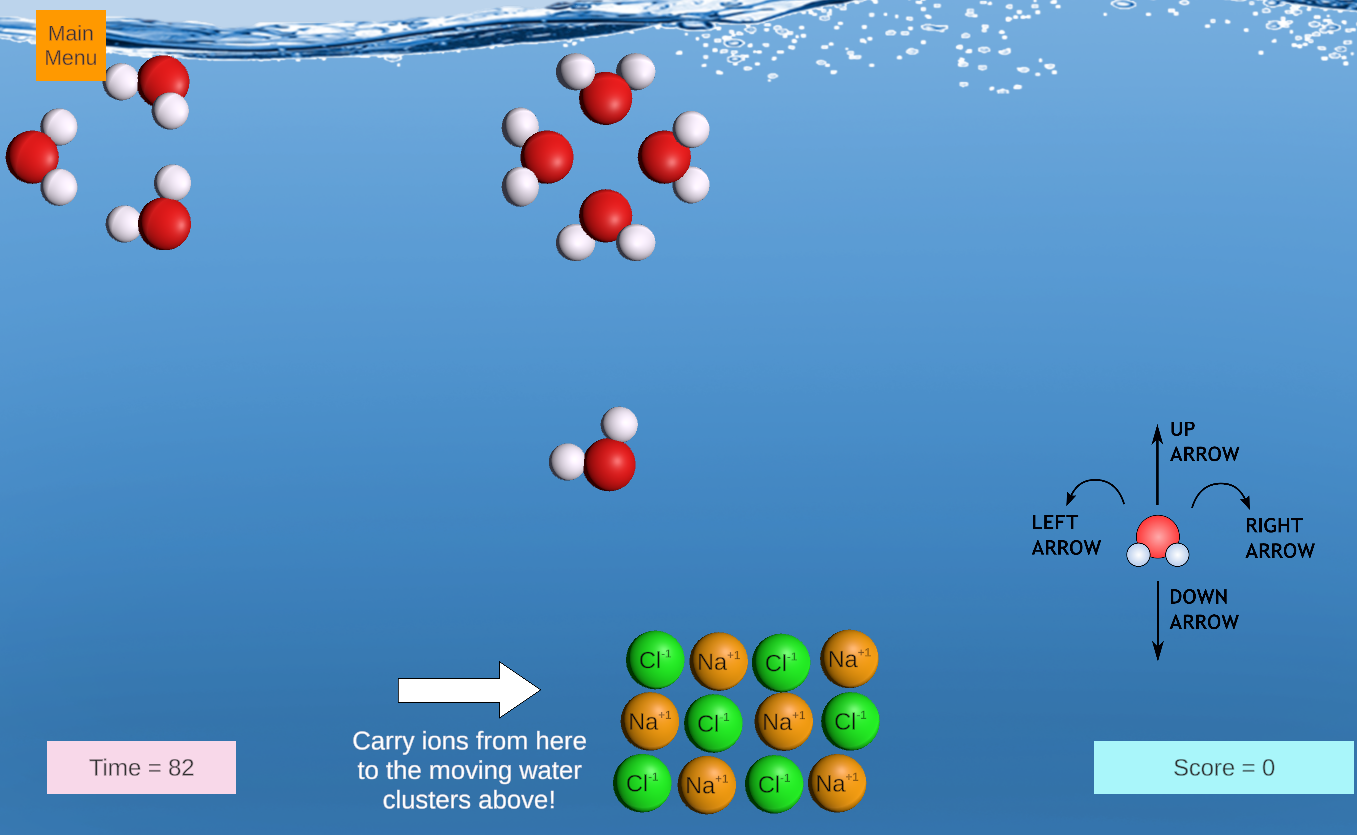
This is an exciting game in which students earn points by steering a water molecule to remove positive and negative ions from a lattice, delivering the ions to moving clusters of water molecules. Salts with limited solubility are introduced in later rounds, leading to concepts of solublity equilibria.
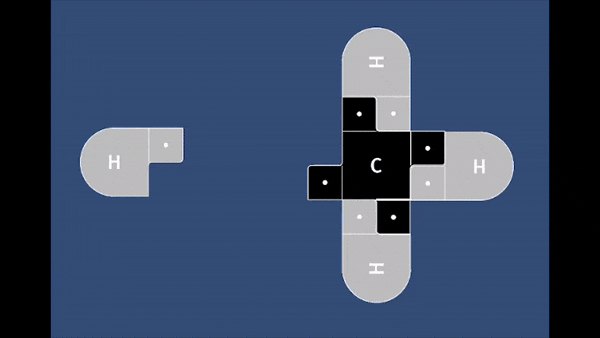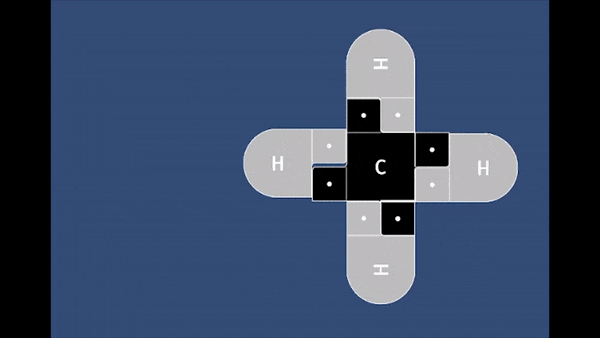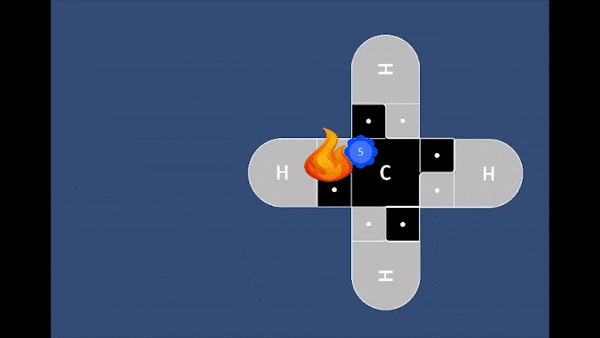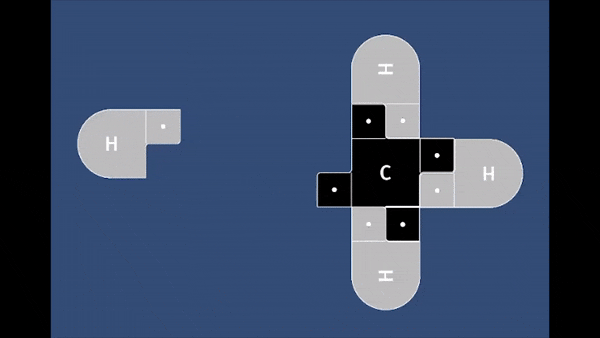
Playable on Mobile Devices! This game allows you to bond atoms together to create molecules using 21st Century Lewis Dot Models. You can build molecules containing carbon, hydrogen, oxygen, nitrogen, and chlorine atoms. The visually pleasing interface is intuitive, and sound effects provide encouragement.
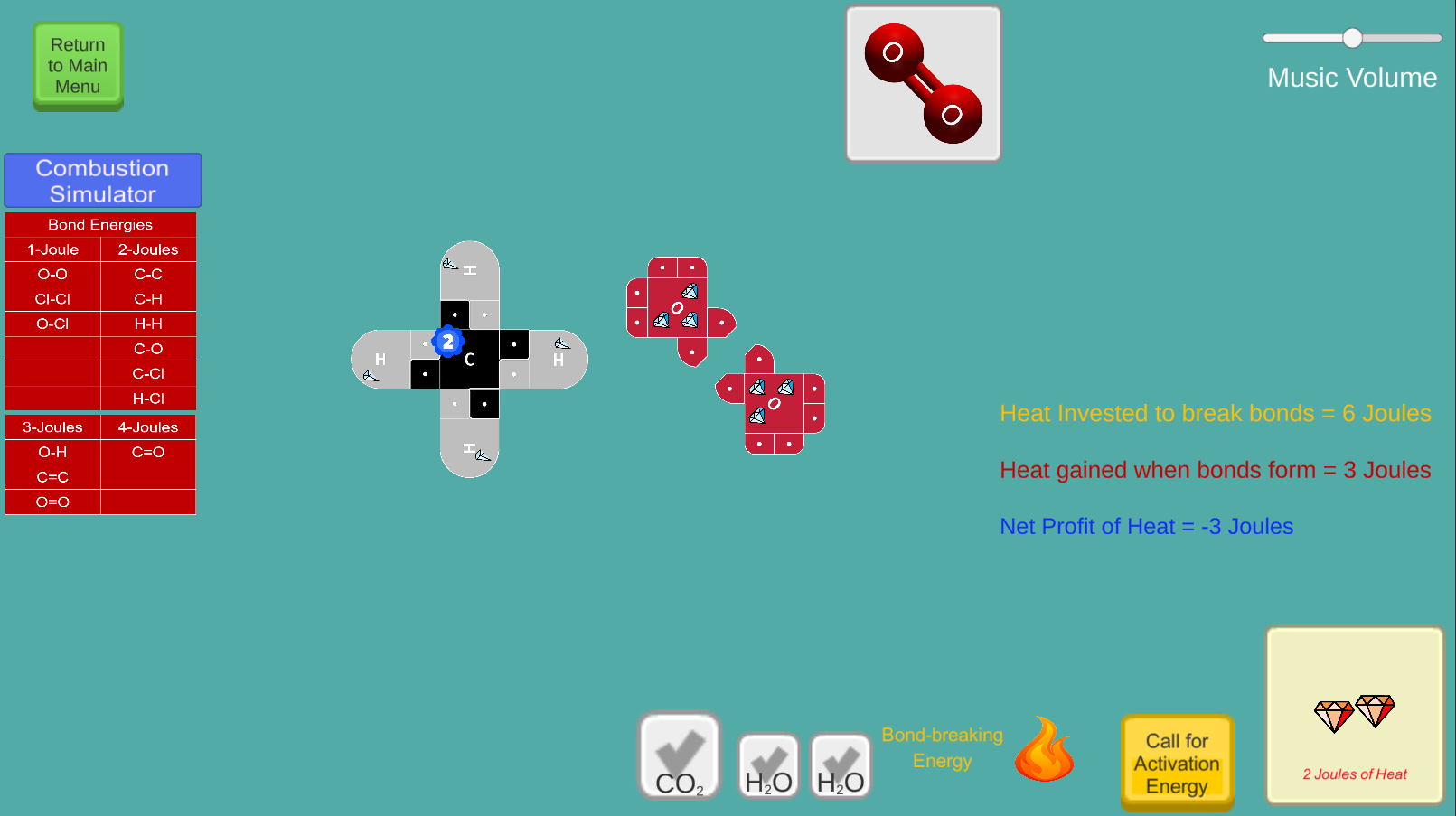
This game simulates combustion reactions involving fuels such as methane and propane. Users apply the concepts of Activation Energy and Conservation of Energy as they invest energy to break bonds and release energy when forming bonds. Molecules are represented using 21st Century Lewis Dot Models to form structures such as CO2 and H2O. Potential Energy in atoms and Heat Energy are made visible using "Jewels" as representations of energy.
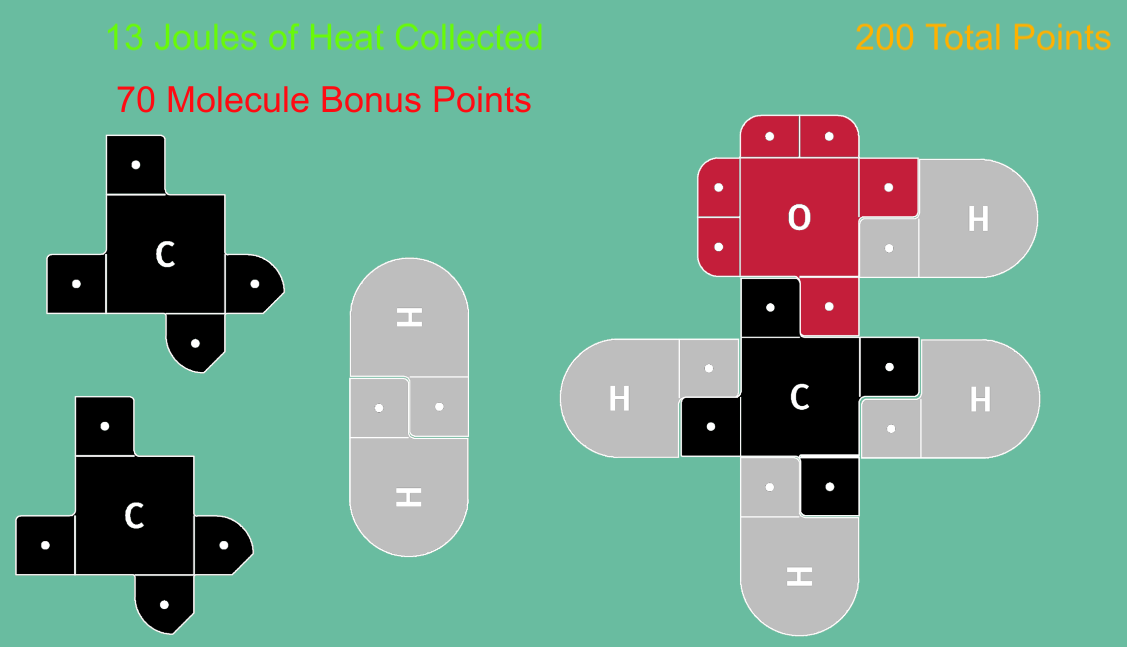
NEW version uploaded February 2026! Now playable on Mobile Devices! This is an exciting game in which students earn points making molecules and gathering "Jewels" of Heat. Using 21st Century Lewis Dot Models, this game is designed to teach students how making and breaking bonds result in energy changes (exothermic and endothermic reactions) We have a few variations on our experiments page: including a Multi-Player version
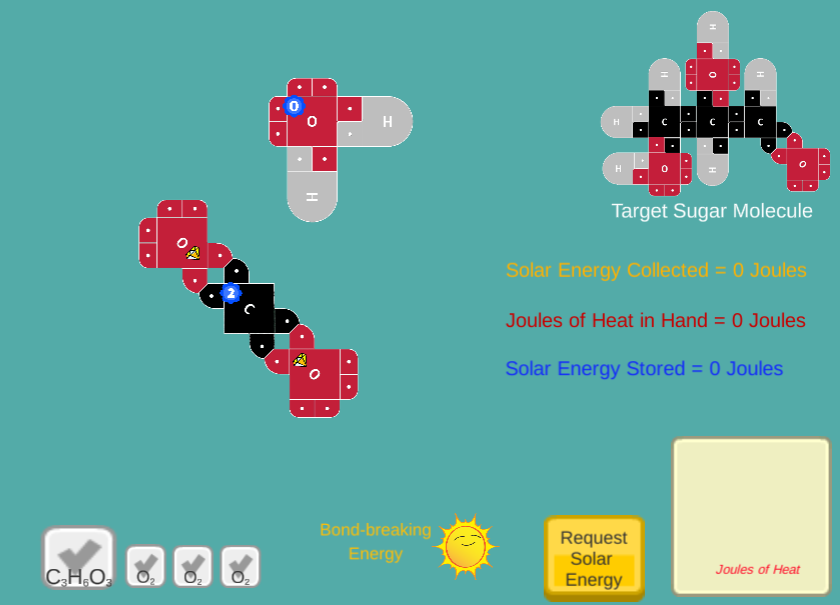
Using 21st century Lewis Dot Models, users will create sugar molecules by adding Joules of Sunlight to CO2 and H2O molecules.
The concept of storing Joules as potential energy in molecules is conveyed using visible Jewels that users can invest to break covalent bonds.
NEW version uploaded December 2024! Now playable on Mobile Devices!
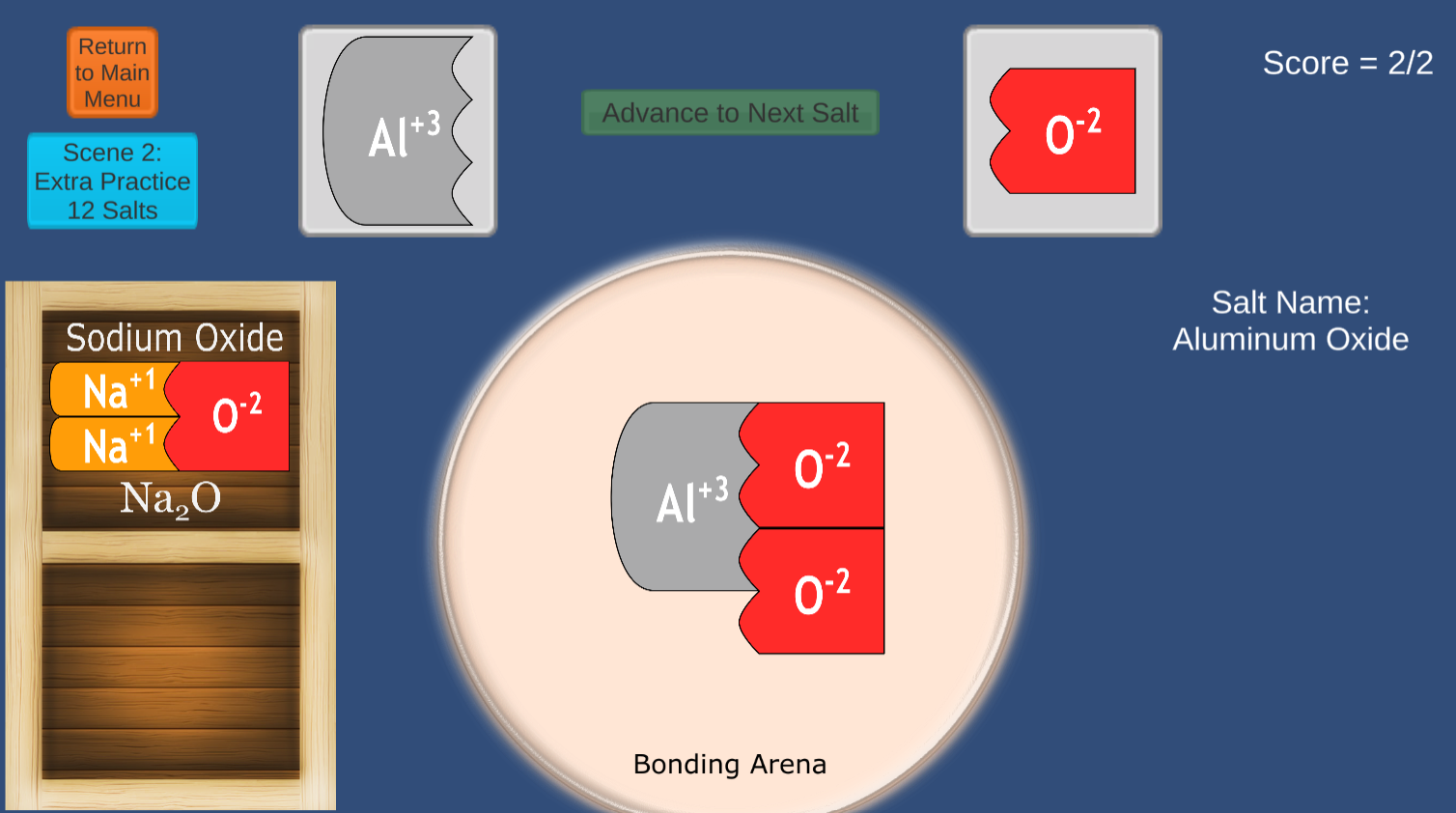
This game will strengthen student skills in creating ionic compounds from common monatomic and polyatomic ions.
The concepts of VALENCE are clearly communicated using colorful interlocking shapes.
The 2024 version offers a Periodic Table game in addition to the classic Matchmaker Game.
Updated in 2025--Now playable on Mobile Devices!
In this game, students use interlocking shapes to model double replacement reactions of ionic compounds.
Students gain practice in writing chemical formulas and balancing chemical equations.
Positive feedback guides students to the correct products in each scene.
This game provides models for the reactions of calcium metal with water and with hydrochloric acid. The transfer of electrons that occurs in these reactions is conveyed using colorful interlocking shapes.
This game allows students to investigate the transfer of electrons and changes in bonding that occur when copper ions react with zinc and aluminum metals. Positive feedback guides students to the correct products in each scene.
In this game, students investigate the ability of catalysts (H+ and an enzyme) to increase the probability of productive bond-breaking collisions. Visible energy meters demonstrate the concept of an energy threshold (Activation Energy) required for reaction. Five scenes offer variations on the type of catalyst and the temperature of the reaction mixture.
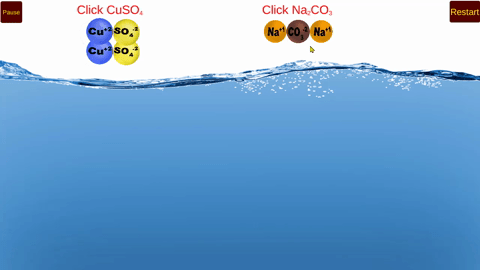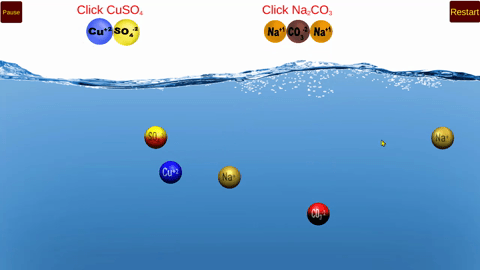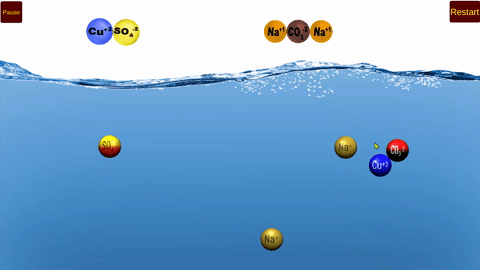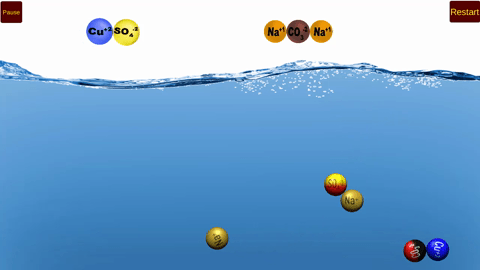
NEW version uploaded May 2025! Now playable on Mobile Devices!
Using this simulator, students will model double replacement reactions that occur in
aqueous solution.
The models clearly illustrate the formation of both precipitates and soluble products!
The concept of excess and limiting reactants are also modeled.
This game provides a rich, entertaining model for electron transfer reactions based on the Activity Series. Users can vary temperature and acid concentration to investigate factors that influence reaction rates.
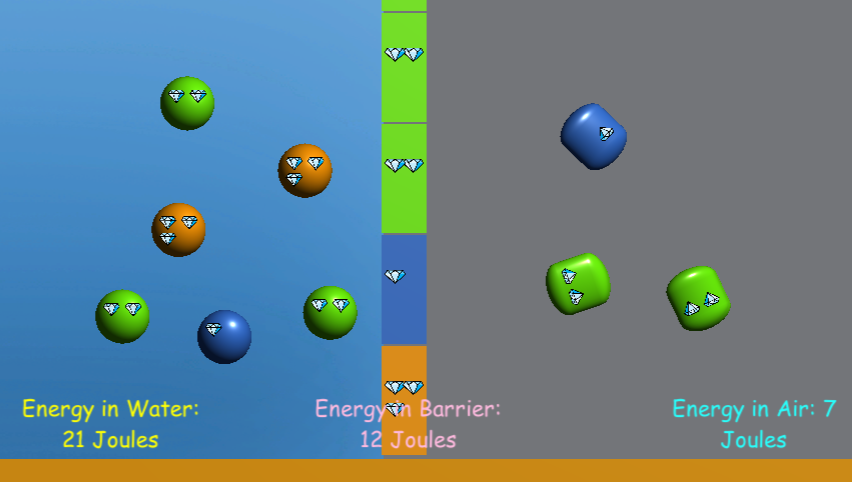
This game will introduce students to the concept of heat transfer through molecular collisions. Several scenarios are offered, emphasizing the concepts of Energy Conservation and Thermal Equilibrium.
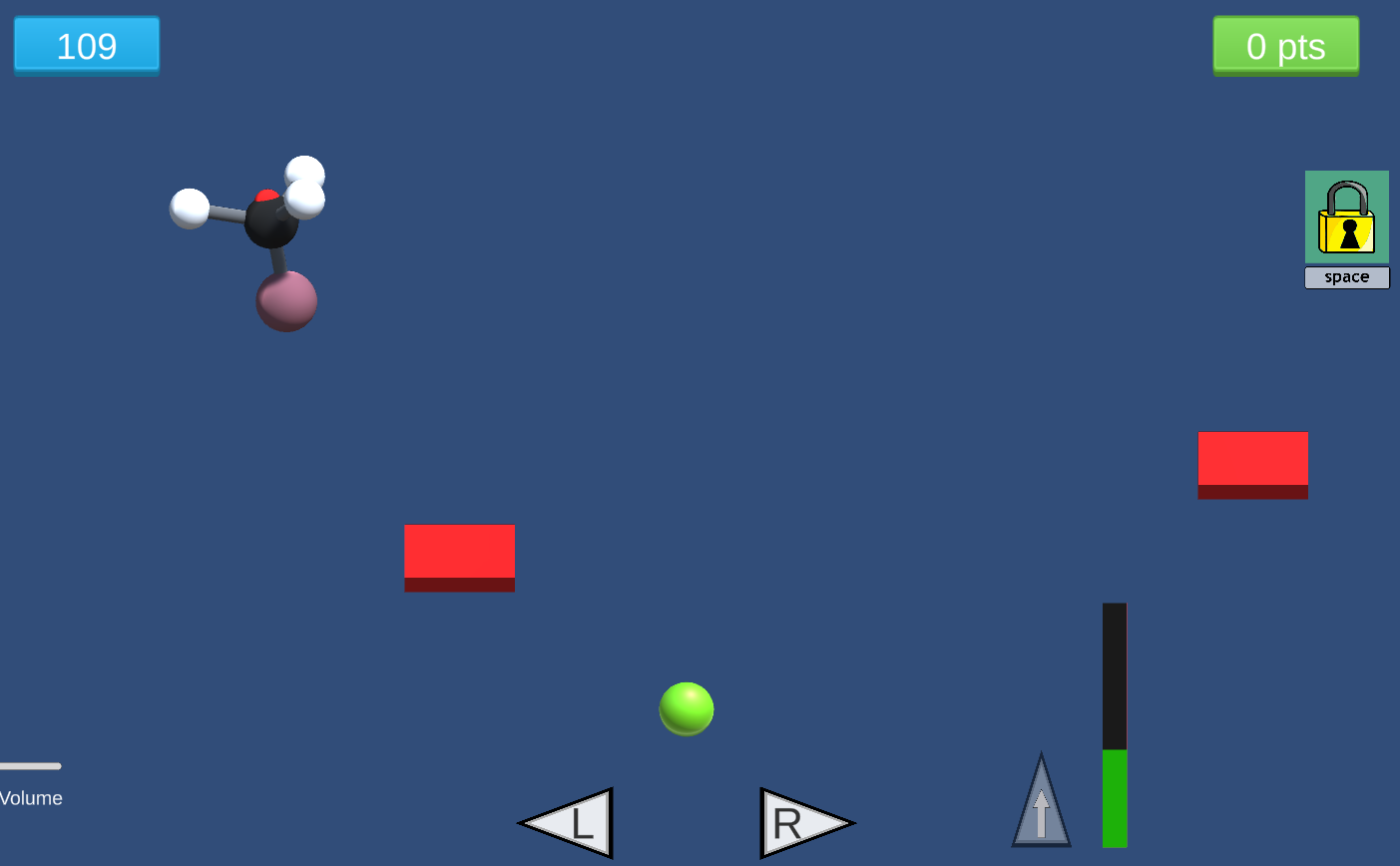
In this game, students investigate nucleophilic substitution reactions (SN2) by firing a nucleophile at a rotating electrophile molecule. Only precisely aligned molecules with sufficient energy can react!
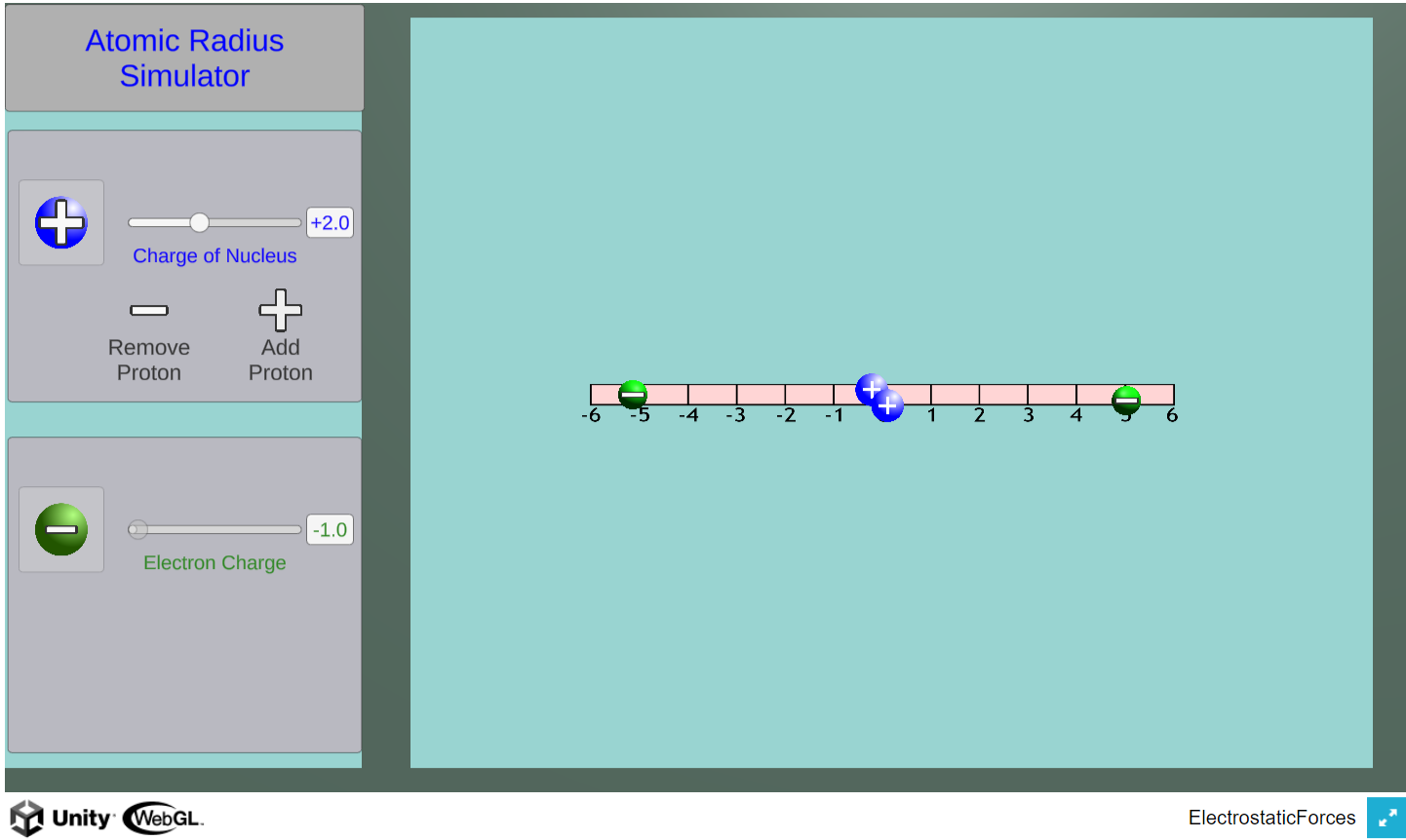
Using this simulator, students can investigate how changes in nuclear charge (number of protons) affects the radius of orbiting electrons.
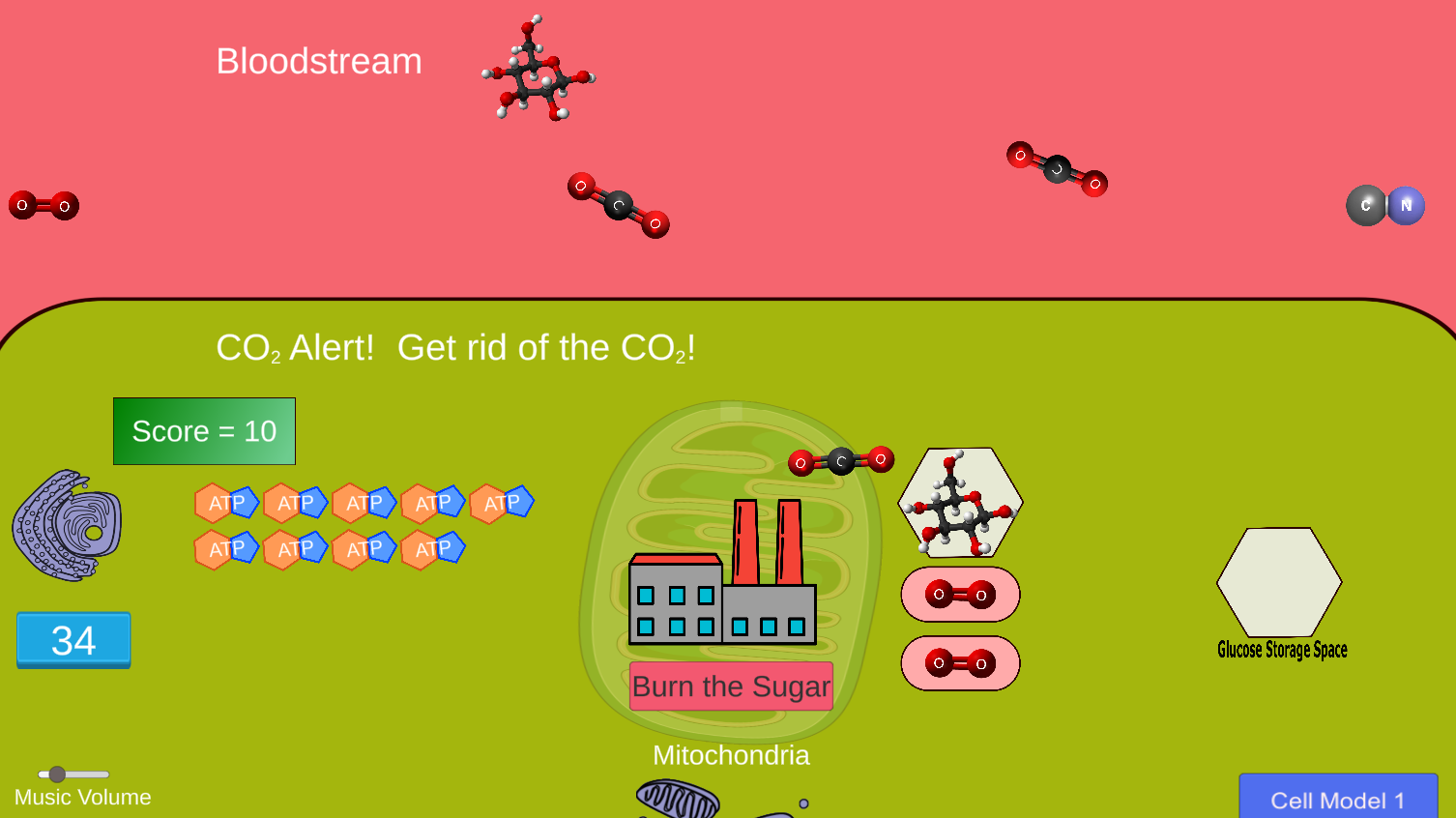
NEW!
In this game, students play the role of a human cell fed by the bloodstream. Players try to import valuable molecules (glucose and oxygen) from the bloodstream while dumping
waste products (carbon dioxide) back into the bloodstream for excretion. The player must maximize ATP production to acheive a high score.
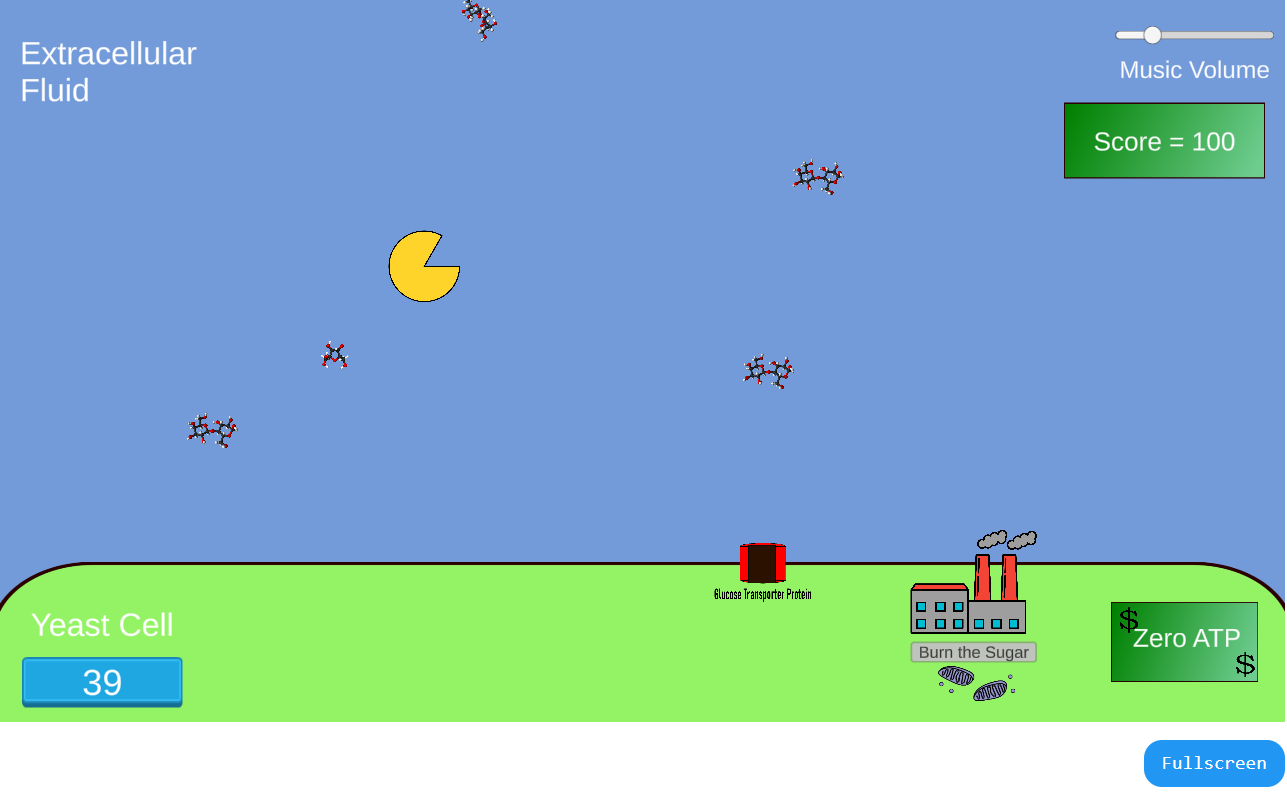
In this game, students play the role of a sucrose-digesting extracellular enzyme (e.g. Yeast invertase). After sucrose is cleaved, a second player steers the
monosaccharides to the glucose transporter on the cell's membrane so the sugars can be internalized.
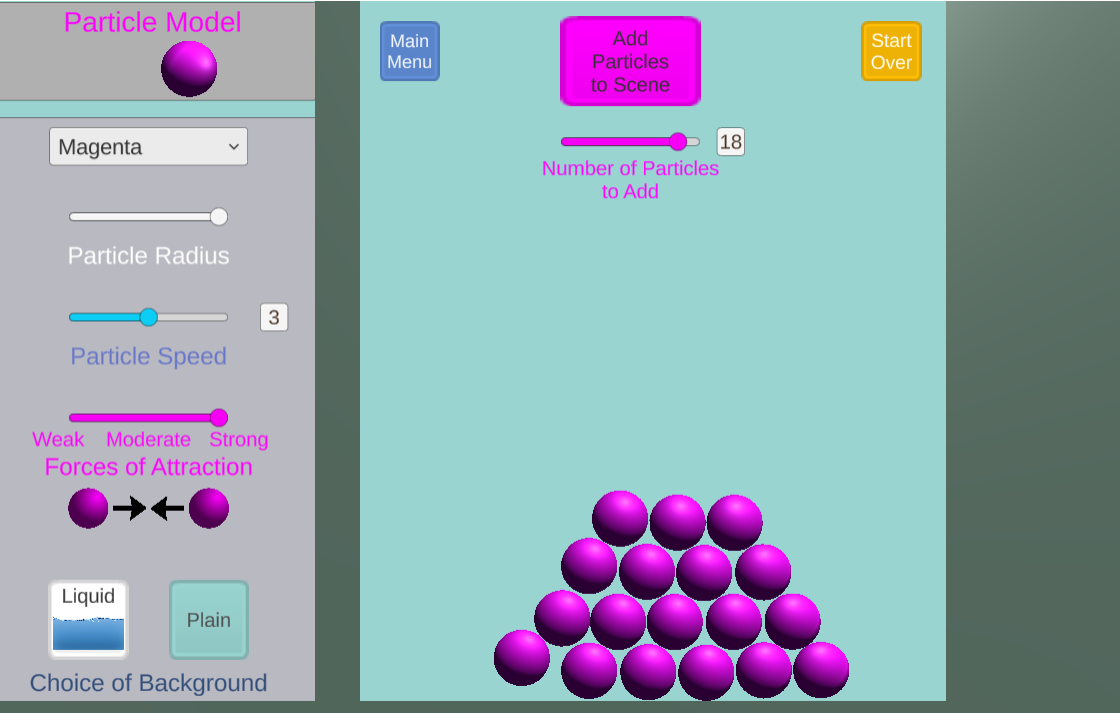
In this game, students investigate states of matter and phase transitions in an open-ended simulation engine. Players can control particle speed, size, and strength of attractions.
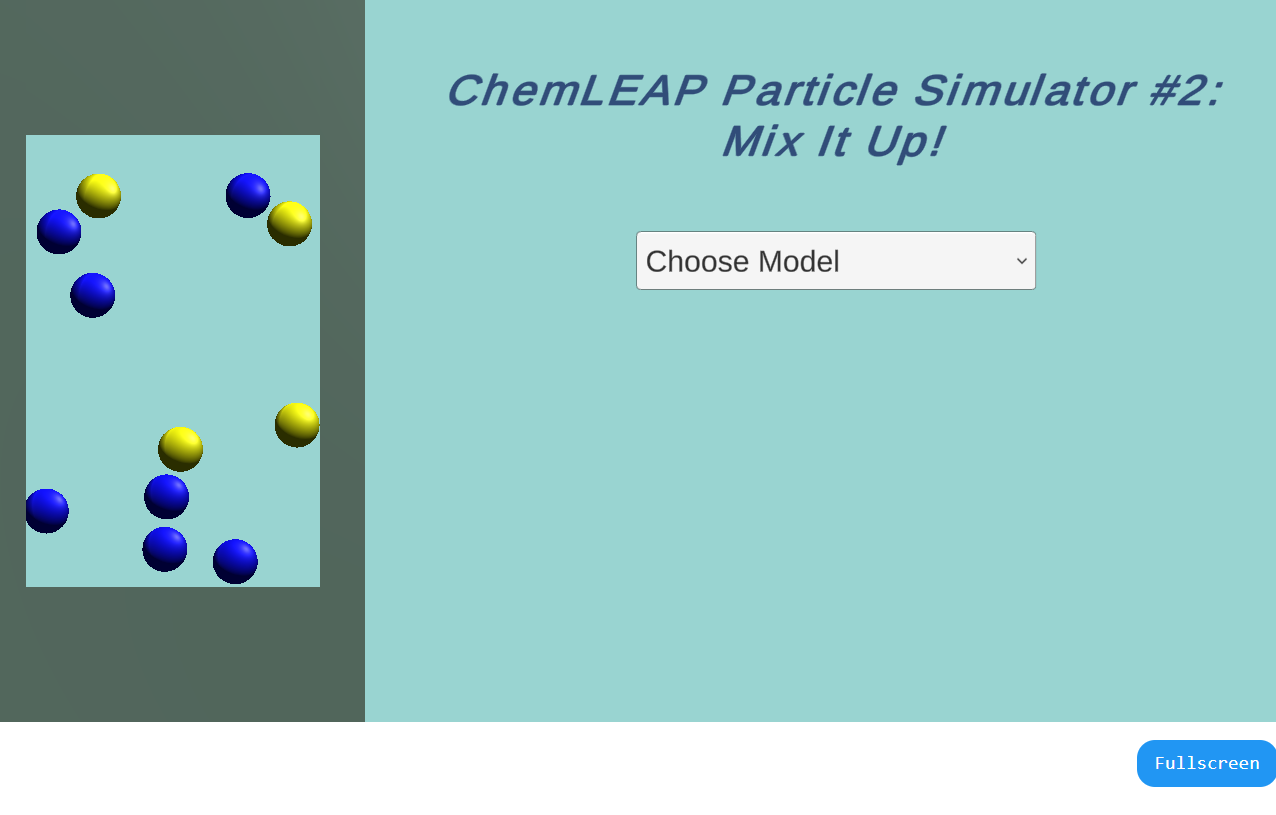
Using this simulator, students can investigate how changes in molecular speed and attractions affect the ability of substances to mix together.
.png)
<
In this game, students will gain a hands-on feel for how Coulomb's Law (attraction and repulsion of charged particles) can move particles in desired directions.
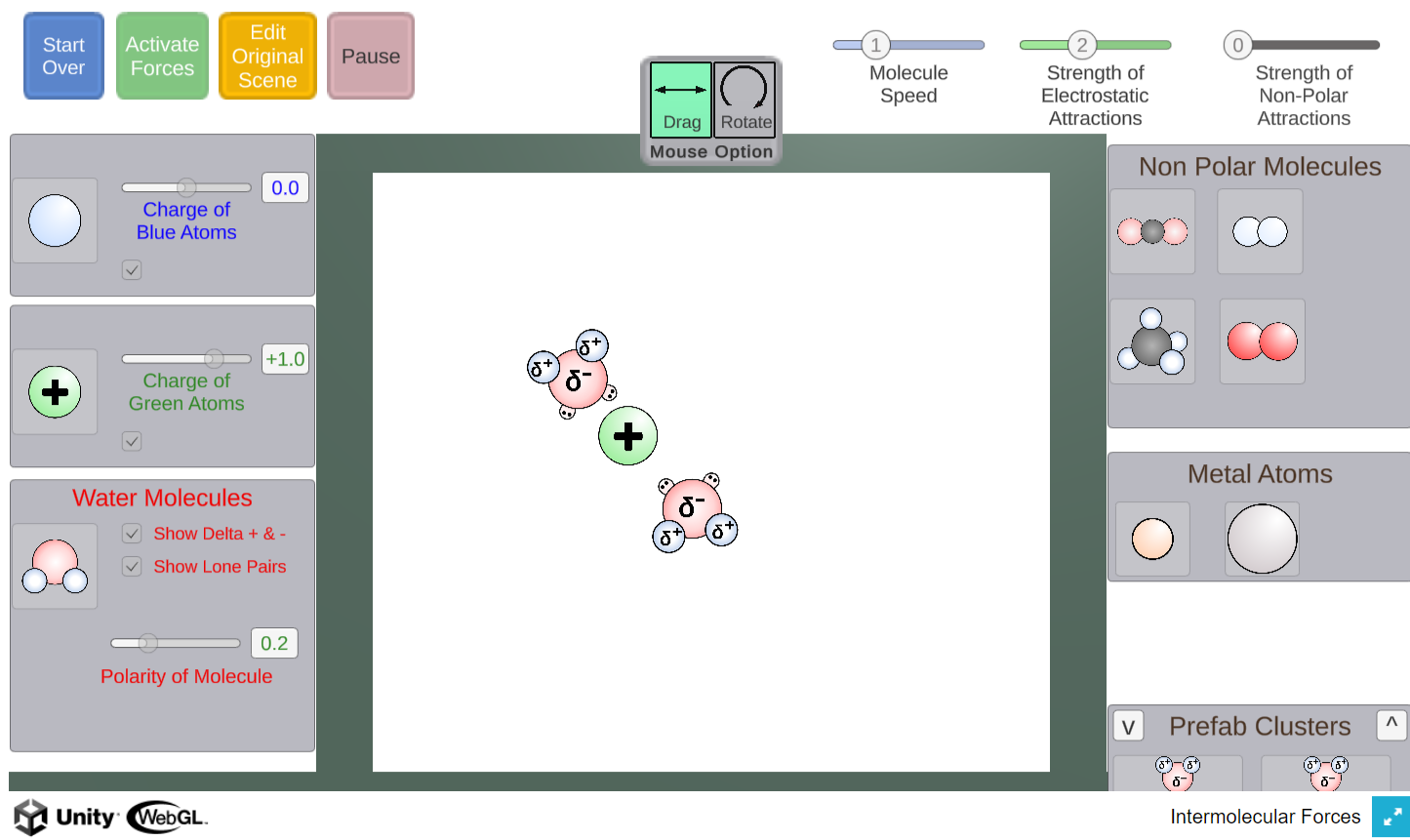
In this simulator, students can create scenarios involving water molecules, ions, metals, and non-polar molecules.
The simulation will then apply appropriate forces allowing students to observe how the particles interact.
On the following page are prototypes and failed experiments that were stable enough to run. Some of these are really cool and may be useful, but you may experience a few bugs and problems. However, if you find a lot of value in one of these projects, you can let us know and we just might finish the project. Otherwise, all the code is on GitHub and you are free to finish the project yourself.
TL;DR These are unfinished projects that might be useful
InteractiveChemistry.org games are created by Chemistry Teacher Steven Sogo, supported by a number of talented high school students at Laguna Beach High School. Our mission is to provide free, open-source games and simulations to help teach principles in chemistry and physics classrooms. Our focus is on web-based applications so that all students can benefit whether they have a Chromebook, Apple Mac, or Windows based device. However, we are interested in new technology like virtual reality. Most of these experiments can be seen on the experiments page. All else can be seen here on the main page. If you have any feedback, want to report any problems with out website, or just want to say "thank you," our contact information is listed at the bottom of the page.